Medical Use, ED Support & HSA/FSA Information
How RX Sleeve Supports Men with ED & Performance Limitations
RX Sleeve products are designed as functional support devices for men experiencing erectile dysfunction or performance limitations due to:
- Erectile dysfunction from various medical causes
- Prostate cancer and related treatments
- Diabetes-related erectile issues
- Medication side effects affecting sexual function
- Hypertension-related difficulties
- Genital injury recovery
- Peyronie's disease
- Post-surgical complications
Our sleeves provide a non-invasive, non-pharmaceutical option for maintaining intimate function. For men who cannot achieve or maintain an erection, our firm material option provides the structural support needed for penetration. For those with partial erectile function, our soft material option works with existing capability.
Unlike medications, RX Sleeve products have no drug interactions, require no waiting period, and can be used regardless of other health conditions that might contraindicate pharmaceutical ED treatments.
Use After Surgery
Many of our customers come to us following medical procedures that have affected erectile function, including:
- Prostatectomy (prostate removal surgery)
- Pelvic surgeries affecting nerve or blood flow
- Penile implant removal or complications
- Cancer treatments in the pelvic region
RX Sleeve provides a way to maintain intimate connection during recovery periods or when surgical outcomes have permanently affected function. Our products are recommended by urologists and therapists who understand that intimacy is an important component of overall wellbeing and recovery.
The large, comfortable loop opening on our harness system accommodates men who have had implants (including those with scrotal pump mechanisms) without causing discomfort or interfering with surgical sites.
FDA Registration
RX Sleeve is an FDA Listed Company manufacturing FDA Class II ED Devices.
FDA listing means our company and products are registered with the U.S. Food and Drug Administration's medical device database. Class II devices are those that require more regulatory control than Class I devices to provide reasonable assurance of safety and effectiveness. This classification places RX Sleeve products in the same regulatory category as other legitimate erectile dysfunction medical devices.
Our products are manufactured using certified "skin safe" platinum silicone that has been independently tested and certified to the OECD TG 439 standard, the internationally recognized protocol for skin safety testing.
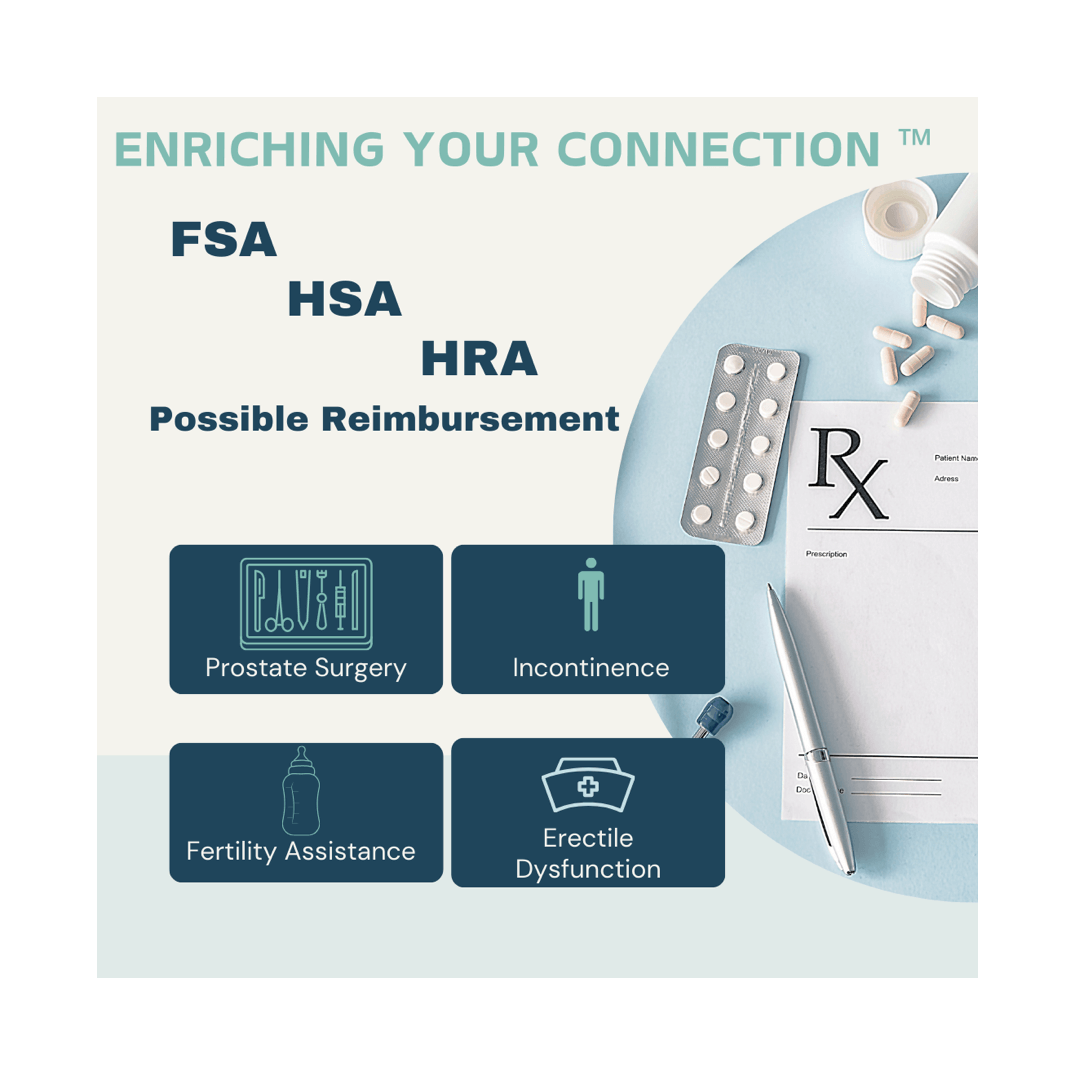
HSA/FSA Eligibility
Can I use my HSA, FSA, or HRA to purchase an RX Sleeve product?
Qualification depends on medical necessity. ED support devices like RX Sleeve products may be eligible expenses under your flexible spending or health savings account when used to treat a diagnosed medical condition.
What you may need:
- A Letter of Medical Necessity (LMN) from your doctor, urologist, or licensed healthcare provider stating that the device is medically necessary for diagnosis, treatment, or management of your condition
- Documentation of your diagnosis (such as ED related to prostate surgery, diabetes, or another qualifying condition)
We provide a downloadable LMN template and sample physician letter to make this process as straightforward as possible.
How to submit your claim:
- Obtain a Letter of Medical Necessity from your healthcare provider
- Keep your RX Sleeve purchase receipt
- Submit both documents to your FSA/HRA/HSA administrator according to their specific procedures
Contact your plan administrator for their exact submission requirements, as these vary by provider.
[Download LMN Template] | [Sample Physician Letter]
Why RX Sleeve Is a Medical Device, Not an Adult Novelty Product
RX Sleeve products are designed, manufactured, and marketed as medical support devices for men with erectile dysfunction and related conditions. Here's what distinguishes us from novelty products:
Medical Purpose & Design
- Engineered specifically to address diagnosed medical conditions (ED, post-surgical complications, performance limitations)
- Designed in consultation with medical understanding of erectile dysfunction
- Recommended by doctors and therapists to their patients
Regulatory Standing
- FDA Listed Company
- Products registered as FDA Class II ED Devices
- Subject to medical device manufacturing standards
Medical-Grade Materials
- Certified skin-safe platinum silicone
- Independently tested to OECD TG 439 certification standards
- Non-porous, hypoallergenic, medical-grade construction
Clinical Positioning
- Sold through medical and healthcare channels
- Marketed to men with medical conditions, not as entertainment products
- Supported by healthcare provider recommendations
Professional Affiliations
- Partnerships with urologists and sex therapists
- Products recommended within VA healthcare systems
- Over 20 years of experience specifically serving men with medical needs
RX Sleeve exists to restore intimate function for men facing real medical challenges, not to provide novelty or entertainment value.